Describe Air Using Mixture Atoms and Molecules
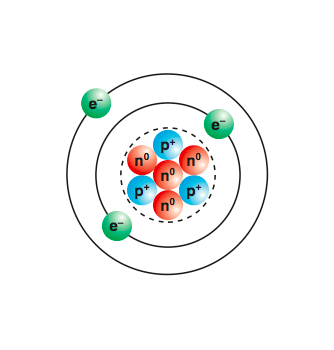
Niels Bohr the developer of the Bohr model of the atom and a leading founder of quantum mechanics. Gases are always around you but the molecules of a gas are much farther apart than the molecules in a liquid.

What Are Atoms Molecules Definition Differences Video Lesson Transcript Study Com
Its all about the physical state and energy in the atoms and molecules.
. It is important to note that R is measured using exhaled gases. The net charge is written with the magnitude before the sign. When writing the chemical formula for an ion its net charge is written in superscript immediately after the chemical structure for the moleculeatom.
Reactivity is dependent upon temperature. Is that a portion of the oxygen metabolized with these foods is required to combine with the excess hydrogen atoms present in their molecules so less carbon dioxide is formed in relation to the oxygen used. Peter Debye used the concept of a molecular dipole to describe asymmetric charge distribution in some molecules.
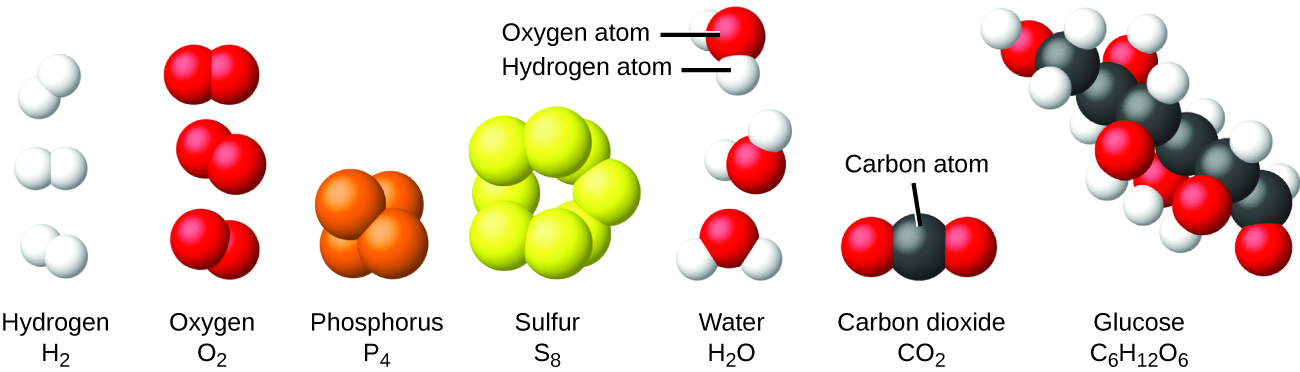
The nitrogen atoms play a pivotal role by subsequently trapping the iron atoms. That is a doubly charged cation is indicated as 2 instead of 2However the magnitude of the charge is omitted for singly charged moleculesatoms. A mixture is a material containing two or more elements or compounds that are in close contact and are mixed in any proportion.
The constituents of a mixture can be separated by physical means like filtration evaporation sublimation and magnetic separation. The transformed iron atoms migrate away from the defects by a hopping mechanism 3233. Cavendish discovered hydrogen as a colorless odourless gas that burns and can form an explosive mixture with air and published a.
When the nitrogen atoms are. They generally burn in water as well as the oxygen in the air. If a gas has an odor youll often be able to smell it.
The most reactive elements and compounds may ignite spontaneously or explosively. For example air sea water crude oil etc. The constituents of a mixture retain.
Physical properties of a solid often include hard and brittle Liquids are fluidy move around a little and fill up containers. Now let us see how one can use the respiratory quotient to determine the relative utilization of different foods. The reaction can involve the substance on its own or with other atoms or compounds generally accompanied by a release of energy.
Atoms Molecules And Compounds Manoa Hawaii Edu Exploringourfluidearth

From Teachwithclass Com Worksheet To Explain Atoms And Molecules With Marshmallows Teaching Chemistry Chemistry Lessons Matter Science
Comments
Post a Comment